Nuclear Lightning and Black Hole Gold
Natures Coolest Atom Factories
There are about a dozen ways that new atoms get made naturally in our universe. Many of you probably know about atoms being made in the Big Bang, or in supernovae, or in the hearts of big stars. And those are interesting things to learn about on a lazy Sunday afternoon. But there are three atomic forges in our universe that I think are special.
Nuclear Lightning
Did you ever see lightning meander across the sky, searching out new paths like incandescent tree roots growing in 1 or 2 seconds? This has to do with how lightning works. Lightning is of course caused by a big difference in charge buildups between clouds and other clouds or between clouds and the ground. This creates a really big voltage difference, and the air is a pretty good insulator, keeping those charge buildups separate. But those charge buildups want to come together and equalize out.
Lightning happens when one of the mountains of charge is able to find a path that gets it closer to the other mountain of charge. The voltage will be so high that it starts to strip electrons off the molecules in the air. When that happens, those stripped-off electrons become free agents, no longer bound to their atoms. Since there’s a big electric field around, those electrons begin to move really fast in the direction of that electric field. They smash into other atoms and heat them up. Now you have a big electric field AND a bunch of heat, both of which make it easier for even more electrons to break free from their atoms.
These electrons become conductors of electricity. In a small tube, maybe a few inches wide, the electrons act like a wire in the air, conducting all the pent up charge across the insulating air. But after moving for only a few dozen feet or so, the field will weaken just enough with distance, and the process will stop abruptly. Why? In the direction of the “wire”, the air at its tip isn’t hot enough or the field isn’t strong enough…and the air stops being a conductive plasma. All those high energy electrons moving crazy fast through the “wire” get stopped super suddenly.
And when you change the velocity of a high-energy charged particle very abruptly, it emits an x-ray or even a gamma ray if the energies and suddenness are strong enough. X-rays are produced in intense showers in a semi-random direction, like a super bright flashlight. Very much like a flashlight, in fact. Because X-rays rays are just super high frequency and thus super high energy photons - light. We cannot see them (lightning is bright due to the aforementioned heating of fast electrons bumping into things, not x-rays which are invisible). But those X-rays are so strong that they disrupt the atoms in the air in that new and random direction, knocking off their electrons. The piled up charge in the nascent lightning bolt then usually chooses to go in that new somewhat-conductive direction, and the whole process repeats. So you have a jump, stop, x-rays in a different direction, new jump, stop, x-rays in a different direction, jump, stop…etc.
This is why lightning sometimes looks like it is moving in little jagged fits and starts across the sky. Cool, right?
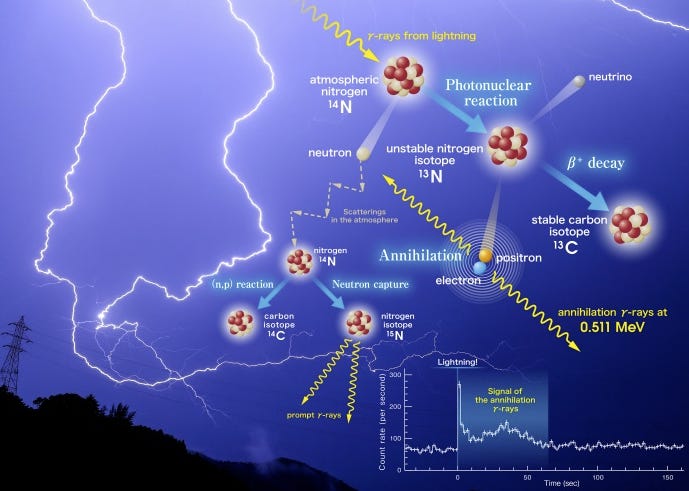
It gets better. The gamma rays are sometimes strong enough to interact not just with the electrons in the atoms, but also the nucleus of a certain atom. Sometimes those gamma rays are so strong they knock a neutron out of a Nitrogen-14 atom, turning it into a Nitrogen-13 atom.
This is a different type of atom, and it is created in an atomic process that occurs in lightning. So most lightning on Earth is Nuclear Lightning. And if that’s not cool enough, they also make antimatter in the form of an anti-electron (a positron).
Atoms From the Edge of the Void
There is a way to make new atoms called the “hot CNO” process. The hot CNO (carbon-nitrogen-oxygen) process occurs whenever you have high enough temperature and a decent proton flux in order to turn carbon into nitrogen and then nitrogen and heavier carbon and then nitrogen and oxygen. The diagram below (start with the Carbon-12 at the top) shows how adding in a hot proton (the H) gets the ball rolling. Protons turn into neutrons by emitting a neutron and some antimatter (positron) and some heat.
This happens in stars like our sun, and is dominant in stars heavier than our sun.
But it also occurs around black holes.
At the accretion disks of black holes, the temperatures of the infalling matter get so high, into the billions of Kelvins, that free protons (hydrogen nuclei) hit nuclei so fast that during the collision they overcome the normal electrical repulsion between positive nuclei and the positive incoming proton. When that happens, when the proton gets within a few femtometers of the nucleus, a different force takes over and latches onto the impinging proton. Imagine pushing together two magnets that repel each other. As they get closer and closer to each other, they repel stronger and stronger. But if you could get them within 1 millimeter of each other, a different force takes over, and they latch with a force 100 times stronger than that magnet repulsion force1. This strong force has been named by physicists “the Strong Force.” This is what keeps protons all bunched up together inside atomic nuclei, even though like charges repel.
This allows the CNO process to occur in the accretion disks of black holes. This might actually account for a significant amount of oxygen production. Sadly, you aren’t breathing any black hole oxygen. The oxygen produced is oxygen-14 and oxygen-15, not the normal and stable oxygen-16 you are breathing right now. Oxygen-14 and -15 decay on average in just a minute or two.
But they decay into nitrogen-14, which is what Nuclear Lightning acts upon. So some nuclei have a very fun life story, perhaps having been forged around a black hole, expelled by titanic magnetic forces from the edge of oblivion, and then formed into Earth’s atmosphere and zapped into nitrogen-13 by Nuclear Lightning.
Further, accretion disks of black holes may also produce enough neutron flux and high temperatures to produce gold. Speaking of which…
Ever Wanted to Touch a Neutron Star?
You probably are already.
A neutron star is the remains of a large star after its outer layers were blasted away. The central region of the star collapsed inward, crushed by its own gravity. The electrons of most atoms within that collapsing core were pressed so hard together by the immense pressure of gravity that they actually merged with the protons in the nucleus, converting them into neutrons. Only near the outer layers do some atoms remain, where the pressure is only ridiculous, not unimaginable.
A neutron star is made mostly of bizarre collections of neutral cores of former atoms, strung together in weird balls, strings, layers. A mass twice that of our Sun is squeezed into a fast-rotating ball about the size of Lower Manhattan, only a few miles across. The gravity is unbelievably intense, only surpassed by black holes. Almost nothing but light can ever escape the surface of a neutron star.
But this is where most of our silver and gold come from.
You see, when normal stars burn and squeeze their innards, they smash together atomic nuclei. That smashing of light atoms, if it forms a heavier element up to iron (with its 56 protons and neutrons), actually releases energy. That energy that gets released is crucial, because it is heat that increases the local pressure in that star’s core. And this pressure is what pushes out the layers of the star, keeping them from falling in due to the star’s own gravity. As big stars burn through (smash together to combine) all their elements lighter than iron, they start to have a lack-of-pressure problem because atoms heavier that iron don’t give out extra thermal energy to help with that pressure when they are produced. So when those lighter atoms run out at the active layers within a star, the star collapses all of a sudden.
A lot of different things can happen based on the mass of the star, but one of the things that can happen is that the core just keeps collapsing into a neutron star. One thing that doesn’t really happen is that the iron doesn’t usually get turned en masse into heavier atoms like silver and gold, which each have 107 and 197 protons and neutrons, respectively2.
On earth, when we want to make heavier atoms, we take what we have and irradiate it in special nuclear reactors that produce a lot of neutrons. As elements absorb neutrons, they slowly — very slowly — transmute into heavier elements with more neutrons and and protons. You can actually turn lead into gold this way. It’s just super slow and expensive.

But wait, how do neutron stars use their neutrons to make silver and gold? And even if they could, how could the silver and gold escape the deep, deep gravity well of a neutron star?
You slam a neutron star with another neutron star.
When neutron stars get close to each other, they orbit in a strong gravitational dance. They would do so almost forever, except this dance produces gravitational waves, periodic stretchings and squeezings of the fabric of spacetime. This slowly takes gravitational (orbital) energy from the neutron star pair. And so, having lost that energy, they orbit closer and closer together. This makes the waves more intense. So intense that we can now detect those waves from billions of light years away as they pass by earth. But those more intense waves are now sapping even more orbital energy from the neutron star pair, and they fall together even faster.
And when they touch and fall into each other, they spray an enormous amount of themselves out in a dense, fast, hot, and violent explosion of neutrons. The remaining atoms on their crust are transmuted in nanoseconds into a wide array of heavier and heavier elements as neutrons are captured from this spray by any atoms3, and new atoms form from the weird neutron layers that are ejected as well. And then those are irradiated by this neutron tsunami, increasing in atomic nuclear size. They escape due to the neutron blast energy. But the rest of the mass of the two stars form either a bigger neutron star, or a black hole.
This happened in our part of the galaxy several times before our solar system formed, and almost all the gold and silver and much of many other elements came from this process. So because a lot of people wear at least a small amount of silver or gold, chances are you are touching pieces of what were once neutron stars right now.
Actually, many of our precious metals and heavier elements came by this hyper-violent process. In the periodic table below, all the purple-shaded elements came mostly from neutron star collisions.

Even more incredibly, sometimes black holes and neutron stars collide. And those collisions might account for up to 30% of our gold.
We’ve only figured out a lot of this in the last 40 years or so. The Nuclear Lightning effect has only been known to science for about 5 years.
I just think it’s cool to know I’m holding a piece of a neutron star or black hole disk that barely escaped spacetime-shattering oblivion.
This doesn’t happen, but imagine if it did.
There are other versions of silver and gold that have more or less neutrons, but 107 and 197 are the most common versions. The different versions of each type of atom is called an “isotope”. Hence the Springfield Isotopes.
If you want to learn more about neutron capture and how it makes heavier atoms, look up the “r-process” of nucleosynthesis.