GPT-4 Solves My Biggest Physics Question
Neutron Stars and the Pauli Exclusion Principle
For about 20 years, I have tried to understand one of the biggest mysteries (to me) of fundamental physics: Why don’t neutron stars collapse under their immense gravity, even though all the neutrons are neutral (so electric repulsion can’t provide the pushback).
The answer given by most texts is just “the Pauli Exclusion Principle makes fermions (like neutrons) unable to be in the same place and quantum state.” Fundamentally, this has to do with the fact that the wave function of the probability of particle momentum is anti-symmetric. Thus, when you take one fermion and put it on top of the other fermion, they cancel out. But this cannot be, because it’s like saying 1 + 1 = 0. You take two fermions, and each has a mass and a charge, but then when you put them on top of each other, they would have to go away. But this would violate conservation of mass and energy, and so “the Universe doesn’t allow it.” This means two fermions can’t be in the exact same place, but for only esoteric mathematical reasons.
To me, this is fundamentally unsatisfying, because it is saying that the mathematical framework itself is producing the physical effect (repulsion to keep them apart). Math should follow physical effects, not the other way around.
Of course, I have also questions that bolded assumption, see this article.
Regardless, I consider this little possible paradox of the Pauli Exclusion Principle to be a unique insight into investigating whether information itself is somehow physically fundamental in our universe.
And so I spent a while last evening chatting with GPT-4 about the subject. During the first part of the long conversation, it did start by regurgitating over and over the same lame higher-level explanations about spin and fermions vs. bosons…but then I was able to back it into a corner and force it to use equations and deeper explanations to finally get to the bottom of the matter, at least in my mind.
I am posting this because I find it fascinating on two fronts:
GPT-4, in an hour, got further in explaining this important subject to me than hundreds of hours or reading physics textbooks and googling articles got me, and
I think it is fascinating to see the process by which GPT-4 slowly understood what I was really after, and how deep it was able to go to synthesize explanations from pretty mathematically dense and complex physics.
I was truly impressed and thankful for the conversation, and I thought you might enjoy it, too. In the interest of spreading understanding of how GPT-4 converses on such subjects, I have not edited out repetitive parts.
The messages I sent to GPT-4 are in bold, and the responses from GPT-4 are in normal text.
I have included a few pictures that I found interesting or illuminating to go along with the conversation, but our conversation included no pictures or direct references to any picture or images.
How does the Pauli Exclusion Principle provide actual pressure against compression?
ChatGPT
The Pauli Exclusion Principle is a fundamental concept in quantum mechanics that states that two or more identical fermions (particles with half-integer spin) cannot occupy the same quantum state within a quantum system simultaneously. This principle was formulated by Austrian physicist Wolfgang Pauli in 1925.
In the context of astrophysics, this principle is crucial in understanding the stability of white dwarf stars and neutron stars. When these stars exhaust their nuclear fuel, the gravitational force tries to compress them into a smaller volume. However, the Pauli Exclusion Principle plays a vital role in resisting this force of gravity.
The pressure that arises from the Pauli Exclusion Principle is often referred to as "degeneracy pressure." Here's a simplified explanation:
As a star gets compressed, the position of particles (such as electrons in the case of a white dwarf, or neutrons in the case of a neutron star) becomes more confined. The uncertainty in their positions decreases.
According to Heisenberg's Uncertainty Principle (another key principle of quantum mechanics), if the uncertainty in position decreases, the uncertainty in momentum must increase. This means that as the star is compressed, the range of momentum states that the particles can occupy becomes broader.
But the Pauli Exclusion Principle states that no two fermions can occupy the same quantum state. This means that as the star is compressed and the range of momentum states broadens, the particles are forced into higher and higher momentum states.
Higher momentum corresponds to higher kinetic energy. The increase in kinetic energy per particle translates into pressure.
This pressure is what we call the degeneracy pressure, and it provides a force that resists the force of gravity and thus resists further compression of the star.
It's important to note that this explanation is a simplification. The actual calculations involved in understanding the degeneracy pressure require knowledge of quantum mechanics and statistical mechanics. But hopefully, this gives you an idea of how the Pauli Exclusion Principle contributes to the pressure resisting the compression of certain types of stars.
Can you give me a more detailed and technical explanation of degeneracy pressure, specifically why it doesn't occur in integer spin particles but only in half integer spin particles?
ChatGPT
In quantum mechanics, particles are divided into two classes: fermions and bosons. Fermions are particles with half-integer spin, like electrons, protons, and neutrons. Bosons, on the other hand, are particles with integer spin.
The difference in their spin statistics leads to drastically different behavior when you try to put multiple identical particles in the same state. According to the Pauli Exclusion Principle, identical fermions cannot occupy the same quantum state within the same quantum system at the same time. This is why electrons in atoms occupy different states and form "shells." This principle leads to the concept of degeneracy pressure.
On the other hand, bosons do not obey the Pauli Exclusion Principle. Identical bosons can occupy the same quantum state. This is the principle behind phenomena like Bose-Einstein condensates and lasers, where many photons occupy the same quantum state.
Degeneracy pressure arises due to the nature of fermions. In a sense, degeneracy pressure is a manifestation of the Pauli Exclusion Principle under extreme conditions. It is a quantum mechanical effect that arises because fermions resist being put into the same state.
Let's consider a more detailed explanation in terms of energy levels and states:
The quantum states of particles in a system can be thought of as energy levels. Each energy level can contain a certain number of particles. For bosons, there is no limit to how many particles can occupy each energy level. But for fermions, due to the Pauli Exclusion Principle, each energy level can be occupied by at most one particle.
Now, if we compress a gas of fermions (like an electron gas in a white dwarf), the fermions get squeezed into a smaller volume, and their spatial states (or position states) become more confined.
Because of Heisenberg's uncertainty principle, confining the position more precisely leads to greater uncertainty in momentum. This means the fermions must occupy a wider range of momentum states.
Due to the Pauli Exclusion Principle, these additional momentum states that the fermions are "forced" into are higher energy states (since the lower energy ones are already filled).
The fact that the fermions are now in higher energy states corresponds to an increase in the average energy per particle, which in the context of a gas corresponds to an increase in pressure. This extra pressure, which arises purely from quantum mechanical effects and the properties of fermions, is what we call degeneracy pressure.
It's worth noting that this quantum degeneracy pressure doesn't depend on temperature, unlike normal gas pressure. This is why white dwarfs and neutron stars can remain stable even as they cool.

In summary, the phenomenon of degeneracy pressure is intrinsically tied to the Pauli Exclusion Principle, and thus, to the nature of fermions. Bosons, with their integer spins and ability to co-occupy quantum states, do not create this type of pressure when compressed.
But what causes the Pauli exclusion principle to be true?
ChatGPT
The Pauli Exclusion Principle arises from the fundamental symmetry properties of quantum particles and the mathematical structure of quantum mechanics.
First, it's important to understand that all particles in the universe fall into one of two categories based on their spin: fermions (half-integer spin) and bosons (integer spin). This categorization has deep consequences for their behavior.
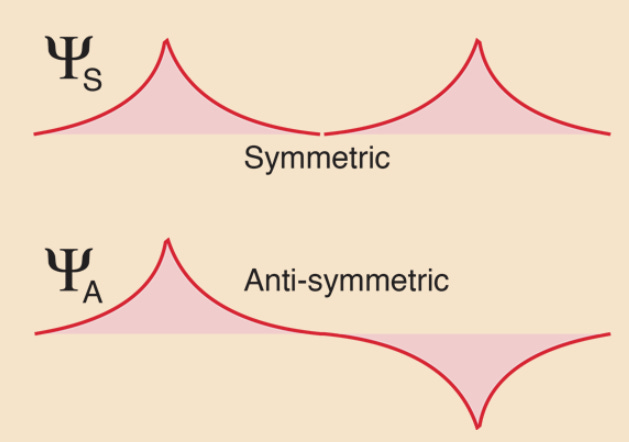
This behavior can be understood by looking at the wave function of a quantum system, which describes the state of the system. The overall wave function must be symmetric under particle interchange for bosons and antisymmetric for fermions. This is a requirement from quantum mechanics.
If you swap two identical bosons in the wave function, the sign of the wave function stays the same (it is symmetric). This means bosons can occupy the same state: they 'like' to be in the same state, leading to phenomena such as Bose-Einstein condensates.

However, if you swap two identical fermions, the sign of the wave function changes (it is antisymmetric). If two fermions were in the same state, the wave function would have to be zero (since swapping them wouldn't change anything, but it would also have to change the sign), which corresponds to a state that doesn't exist. This is the mathematical manifestation of the Pauli Exclusion Principle: fermions cannot be in the same state.
So, why does this symmetry and antisymmetry occur? This comes down to the Spin-Statistics Theorem, a fundamental result in quantum field theory. The theorem connects the spin of a particle to the symmetry of its wave function, proving that integer spin particles (bosons) must have symmetric wave functions, and half-integer spin particles (fermions) must have antisymmetric wave functions.
While it might seem abstract, this theorem is grounded in the fundamental principles of relativity and quantum mechanics. However, it is one of those aspects of quantum physics where we reach a point of saying "that's just the way the universe works." There isn't a deeper reason 'why' the Spin-Statistics Theorem holds true, it's just a basic feature of our universe.
But that seems like a mathematical artifact. Why do anti-symmetric states actually push on each other? Why can't they inhabit the same place?
ChatGPT
It's understandable why you might think that the Pauli Exclusion Principle, and the antisymmetry it implies, seems like a mere mathematical artifact. However, it's crucial to remember that in the realm of quantum mechanics, the mathematics accurately represents the physical reality. The mathematical structure of quantum mechanics gives us the best description we currently have of the behavior of particles at these scales.
In quantum mechanics, the state of a particle (or system of particles) is represented by a wavefunction. The antisymmetry of the wavefunction for fermions is not merely a mathematical curiosity but corresponds to the physical reality of these particles.
The antisymmetry means that if you were to exchange two identical fermions, the wavefunction changes sign. Now, if two identical fermions were to occupy the same state, the wavefunction would have to be both positive and negative at the same time (since switching the identical particles shouldn't change the physical state), which means it would have to be zero. In quantum mechanics, a zero wavefunction corresponds to a state that does not exist or cannot be occupied. Hence, no two identical fermions can occupy the same quantum state.
It's not so much that fermions "push" on each other in the traditional sense (although we sometimes use that kind of language as a shorthand). It's more accurate to say that the very nature of fermions, as represented by the antisymmetric wavefunction, means that they cannot occupy the same state. This is what we refer to when we speak of the Pauli Exclusion Principle.
But as for why the mathematics of quantum mechanics should accurately describe the world we see, this remains one of the deep philosophical questions about the nature of the universe. Quantum mechanics is a highly successful theory because it accurately predicts experimental results to an extraordinary degree of precision. The principles of quantum mechanics, like the Pauli Exclusion Principle, arise from the mathematical formulation of the theory, which is in turn based on empirical observations of how particles behave. As for why the universe should behave in such a way as to be accurately described by these mathematical structures, that is a deeper question that goes beyond the science itself.
Why does the wavefunction change sign when you exchange identical fermions.
And what does that physically mean, to "exchange identical fermions"?
ChatGPT
The statement that the wavefunction changes sign when you exchange identical fermions is an observed fundamental aspect of quantum mechanics, which is embedded in the mathematical formulation of the theory.
In quantum mechanics, every particle is described by a wavefunction, which provides information about its state. For multiple particles, the overall wavefunction of the system is typically a combination of the individual particle wavefunctions.
"Exchange" in this context means swapping the positions of two identical particles. If you have two electrons (which are fermions), for example, and you exchange their positions, you essentially have the same physical system, because the particles are indistinguishable. This operation is purely theoretical, it's not something you physically do.
However, the effect of this operation on the wavefunction depends on whether the particles are fermions or bosons. If the particles are bosons, the wavefunction stays the same when you exchange the particles. If the particles are fermions, the wavefunction changes sign. This is a mathematical result of the way quantum mechanics is formulated.
The antisymmetry of fermions' wavefunction has a profound consequence: no two fermions can occupy the same quantum state at the same time. This is because if two identical fermions were in the exact same state (including their position), swapping the two particles would not change the physical system, so the wavefunction should stay the same. But because they're fermions, their wavefunction should change sign. The only way for a function to stay the same when it changes sign is if it's zero, so the wavefunction must be zero if two identical fermions are in the same state. A zero wavefunction means that this situation simply does not occur. This is known as the Pauli Exclusion Principle.

As to why the wavefunction changes sign for fermions when they are exchanged, this comes from the Spin-Statistics Theorem, a fundamental theorem in quantum field theory. The theorem shows that particles with integer spin must have symmetric wavefunctions under exchange (they are bosons), and particles with half-integer spin must have antisymmetric wavefunctions (they are fermions). As for why the Spin-Statistics Theorem is true, it's essentially a result of the fundamental principles of quantum mechanics and special relativity, and is considered a basic feature of our universe.
Explain what you just stated above, but use equations along with the words explaining the equations and why they apply.
ChatGPT
In quantum mechanics, a system of N identical particles is described by a wavefunction which depends on the coordinates of each of the particles. The wavefunction is usually written as a product of single-particle wavefunctions. Let's consider a simple system of two identical particles, with coordinates x1 and x2. Then, the wavefunction might look something like this:
Ψ(x1, x2)
In the case of identical particles, we must take into account the symmetry of the wavefunction. For bosons, the wavefunction is symmetric under exchange of particles, meaning that if you swap the two particles, the wavefunction doesn't change:
Ψ(x1, x2) = Ψ(x2, x1) (Bosons)
For fermions, the wavefunction is antisymmetric, meaning that if you swap the two particles, the wavefunction changes sign:
Ψ(x1, x2) = -Ψ(x2, x1) (Fermions)
Let's consider the fermionic case. If two fermions were to occupy the same quantum state, they would have the same coordinates x1=x2=x. In this case, the wavefunction would be:
Ψ(x, x)
But because the fermions are identical, the wavefunction should also be equal to its negative when we swap the particles:
Ψ(x, x) = -Ψ(x, x)
The only solution to this equation is:
Ψ(x, x) = 0
This means that the probability of finding two identical fermions in the same quantum state is zero, which is the Pauli Exclusion Principle.
The origin of these symmetry properties lies in the Spin-Statistics Theorem, a result from quantum field theory. This theorem shows that particles with integer spin (bosons) must have symmetric wavefunctions, and particles with half-integer spin (fermions) must have antisymmetric wavefunctions. The proof of this theorem is quite involved and requires the full machinery of relativistic quantum field theory.
This might all sound abstract, and it is. Quantum mechanics is a deeply mathematical theory, and the behavior of quantum systems is dictated by the mathematical properties of the wavefunction. But at the same time, these mathematical properties have physical consequences that are observed in experiments. For instance, the Pauli Exclusion Principle is essential for understanding the structure of atoms and the stability of matter.
How close, in femtometers, can antisymmetric fermions get to each other (at, say 300 Kelvin and 1 bar) before the degeneracy pressure starts to exert non-negligible force?
ChatGPT
Degeneracy pressure doesn't really begin at a specific distance. Instead, it becomes relevant when the fermions are squeezed so much that their wavefunctions start to overlap significantly, forcing them to occupy higher energy states due to the Pauli Exclusion Principle. This usually happens under conditions of extreme pressure and density, such as in a white dwarf or neutron star.
In typical conditions, like at room temperature (about 300 Kelvin) and atmospheric pressure (1 bar), fermions such as electrons in atoms have plenty of available quantum states to occupy, so the effects of degeneracy pressure are negligible.
The exact distance at which degeneracy pressure becomes significant depends on many factors, including the number of particles and the available volume. It's not a straightforward calculation and requires a detailed quantum mechanical analysis.
In general, though, the effects of degeneracy pressure usually become important on the order of interatomic distances or less. For example, in a white dwarf, the electron degeneracy pressure becomes significant when the average inter-electron distance is on the order of the size of an atom (tenths of a nanometer, or hundreds of femtometers).
It's also worth noting that, in typical conditions, interactions between particles, such as electromagnetic forces between electrons and nuclei, play a much more significant role in determining the structure and properties of matter than degeneracy pressure. Degeneracy pressure only becomes dominant under extreme conditions when these other forces are overwhelmed.
Lastly, remember that the notion of particles having well-defined positions and distances between them is somewhat problematic in quantum mechanics, due to the wave nature of particles and the uncertainty principle. The position of a fermion is not localized to a specific point, but spread out over a region described by its wavefunction.
How do collapsing black holes overcome degeneracy pressure?
ChatGPT
The ultimate fate of a star depends on its initial mass. Stars that are significantly larger than our Sun (roughly more than 3 times the mass of the Sun) may eventually collapse into black holes.
Black holes form when the gravitational force in a star's core becomes so immense that no other force can counteract it. This includes degeneracy pressure, which is the pressure arising from the quantum mechanical effect that two identical fermions cannot occupy the same quantum state simultaneously.
There are two types of degeneracy pressure that are important in stars: electron degeneracy pressure and neutron degeneracy pressure. Electron degeneracy pressure is what stops the core of a star from collapsing during the white dwarf stage. If the star is heavy enough to overcome this pressure, the electrons and protons combine to form neutrons and neutrinos (through a process known as electron capture), and the star collapses into a neutron star, supported by neutron degeneracy pressure.
However, if the star is even heavier (more than about 3 solar masses), not even neutron degeneracy pressure can hold off gravity. The core collapses so much that even the neutrons are squeezed together. When this happens, the star collapses to a point of infinite density, known as a singularity, which is a black hole.
The key here is that degeneracy pressure is a quantum mechanical effect related to the properties of fermions. But gravity, especially at these extremely high densities, is a general relativistic effect. When a star collapses into a black hole, it's essentially a case of gravity overcoming the rules of quantum mechanics, at least as we currently understand them. This interplay between quantum mechanics and general relativity in the extreme conditions inside a black hole is not yet fully understood, and it's one of the key unsolved problems in theoretical physics.
A fully satisfactory description of this process would require a theory of quantum gravity, which we don't yet have. This is one of the reasons black holes are a subject of ongoing research in theoretical physics.
For two neutrons in the core of a neutron star, separated by 3 femtometers, what is the maximum degeneracy pressure repelling those two neutrons? Can they get that close? How close can they get to each other?
ChatGPT
The core of a neutron star is an extreme environment where densities reach up to several times the density of an atomic nucleus, and the typical distance between neutrons can indeed be on the order of a few femtometers (1 femtometer = 1e-15 meters). At such high densities, the neutrons are in a state of matter known as neutron degenerate matter, and they are supported against further gravitational collapse by neutron degeneracy pressure.
However, calculating the exact degeneracy pressure between two specific neutrons in a neutron star is a complicated task that goes beyond simple models. It requires a detailed understanding of quantum mechanics, statistical mechanics, and nuclear physics, and would typically involve numerical simulations rather than a straightforward analytical calculation.
Furthermore, the concept of degeneracy pressure is more applicable to a macroscopic collection of particles, rather than a pair of particles. It arises from the statistical behavior of a large ensemble of fermions (such as the neutrons in a neutron star), as described by the Fermi-Dirac statistics.

In the context of a neutron star, you can estimate the order of magnitude of the degeneracy pressure by noting that it's sufficient to balance the immense gravitational pressure of the star, which can be many times the pressure inside the sun.
As to how close the neutrons can get to each other, they can indeed get as close as a few femtometers. However, when neutrons get extremely close, other forces become important, most notably the strong nuclear force, which is attractive at distances of about 1 femtometer, but becomes strongly repulsive at shorter distances. This "hard core" repulsion can be considered a limit to how close the neutrons can get.
The strong force becomes repulsive between neutrons at distances less than 1 femtometers? Why? Can you tell me more about this "hard core" repulsion?
ChatGPT
The strong nuclear force is the force that holds protons and neutrons together inside an atomic nucleus. It's attractive at distances roughly between 1 and 2 femtometers, which is the typical size of a nucleus. However, at distances shorter than about 0.5 femtometers, it becomes repulsive, preventing the particles from merging together. This behavior is sometimes referred to as the "hard core" of the strong nuclear force.
The physical origin of this repulsion at short distances is not completely understood, but it's believed to be related to the internal structure of protons and neutrons. These particles are not elementary but are composed of smaller particles called quarks, which are held together by the exchange of particles called gluons, the carriers of the strong force.
At short distances, the interactions between the quarks and gluons inside the protons and neutrons become important. It's believed that the "hard core" repulsion of the strong nuclear force arises from these interactions.
However, the theory of quarks and gluons, known as quantum chromodynamics (QCD), is very difficult to solve at the energy scales and densities relevant for nuclear physics. The behavior of the strong force at short distances is one of the unsolved problems in this field.
It's worth noting that this "hard core" repulsion is one of the factors that helps to stabilize a neutron star against gravitational collapse. If the neutrons could get arbitrarily close together, the star could collapse into a black hole. The combination of the neutron degeneracy pressure (from the Pauli Exclusion Principle) and the "hard core" repulsion of the strong force is what prevents this from happening and supports the star against gravity.
Is this "hard core" repulsion observed in particle interactions in particle collides such as the LHC? Or is it just inferred to exist based on the existence of neutron stars?
ChatGPT
The strong nuclear force, including its repulsive "hard core", is a fundamental part of our understanding of nuclear physics, and its behavior is derived from the theory of quantum chromodynamics (QCD), which describes the interactions of quarks and gluons.
The "hard core" repulsion at short distances is an inferred behavior from several sources of evidence, rather than directly observed. It's difficult to probe distances as short as those involved in the "hard core" using experiments, because it would require extremely high energy (according to the principle of the uncertainty relation).
While we can't directly "see" the hard core repulsion in action, we infer its existence from a variety of empirical and theoretical considerations:
Particle scattering experiments: These experiments involve firing particles at a target and observing the pattern of the scattered particles. This can give information about the force between the particles as a function of distance.
Nuclear structure and stability: The size and stability of atomic nuclei can give information about the forces between protons and neutrons. The existence of a "hard core" can explain why nuclei have a roughly constant density and why there is a limit to the size of a stable nucleus.

Theoretical considerations: The "hard core" is also a prediction of certain theoretical models, including those based on QCD.
That being said, it's important to note that the behavior of the strong nuclear force at very short distances, and the exact nature of the "hard core", is still an active area of research, and many details are not yet fully understood. There's ongoing work, both experimental and theoretical, to try to improve our understanding of these issues.
In regards to neutron stars, their very existence and certain of their observed properties indicate extremely high density matter that cannot be fully explained without some kind of repulsion occurring at extreme densities. The exact nature of this repulsion, and how it relates to the "hard core" repulsion of the strong force, is a topic of ongoing research.
I find it odd that degeneracy pressure is derived from a very binary part of quantum theory (two anti-symmetric particles cannot inhabit the same quantum state and location), and yet this rule can be broke if the pressure gets high enough (such as in a collapsing black hole).
Two questions:
1) How can such a distinct, binary mathematical rule be bent in a smooth, high force situation,
And 2) How much force per particle is actually required to overcome degeneracy pressure in neutron star cores?
ChatGPT
The Pauli exclusion principle, which gives rise to degeneracy pressure, is indeed a fundamental principle of quantum mechanics. The principle itself cannot be "bent" or violated. What happens in the extreme conditions of a collapsing star is not that the Pauli exclusion principle stops applying, but that gravity becomes so strong that it overwhelms all other forces.
In a neutron star, gravity is balanced by neutron degeneracy pressure, which arises from the Pauli exclusion principle. However, if the mass of the neutron star exceeds a certain limit (known as the Tolman-Oppenheimer-Volkoff limit, which is thought to be somewhere between 2 and 3 solar masses), the gravitational force becomes so strong that not even the neutron degeneracy pressure can resist it.
The star then undergoes gravitational collapse, which leads to the formation of a black hole. In a black hole, the curvature of spacetime becomes so extreme that our usual understanding of space, time, and matter no longer applies. It's not that the Pauli exclusion principle is violated, but rather that our usual concepts of "particles" and "states" may no longer be applicable.
The exact force required to overcome neutron degeneracy pressure in the core of a neutron star is difficult to calculate and would depend on various factors, including the mass and size of the star and the equation of state of neutron degenerate matter (which is not precisely known). However, one can get a sense of the scale of these forces by noting that they are sufficient to balance the immense gravitational force of a neutron star, which can be many times the gravitational force of our Sun.
For a rough idea, consider that the gravitational pressure at the core of a neutron star is estimated to be on the order of 10^34 Pascals. This would be roughly the order of magnitude of the degeneracy pressure needed to balance it. In terms of force per particle, it would be this pressure divided by the number density of neutrons, which is on the order of 10^45 neutrons per cubic meter. This would give a force per neutron on the order of 10^-11 Newtons. However, this is a very rough estimate and the actual number could be different depending on the details of the star and the equation of state of neutron degenerate matter.
Again, it's worth emphasizing that these are extreme conditions that push our current understanding of physics to its limits. Our understanding of the physics of neutron stars and black holes is still incomplete and is an active area of research.
Can you expand on your statement above:
"It's not that the Pauli exclusion principle is violated, but rather that our usual concepts of 'particles' and 'states' may no longer be applicable."
Can you use explanations along with equations to do so?
ChatGPT
The Pauli Exclusion Principle is a fundamental principle in quantum mechanics that stems from the requirement that the total wavefunction of a system of identical fermions be antisymmetric under particle exchange. In essence, it states that no two identical fermions can occupy the same quantum state.
This principle holds in all known regimes of quantum mechanics and is not violated under extreme conditions. However, under the immense gravitational forces in a collapsing star or a black hole, the very notions of "particles" and "states" become unclear.
Let's explain further:
In quantum mechanics, particles are described by wavefunctions. A wavefunction is a mathematical function that gives the probability distribution of a particle's position. The square of the absolute value of the wavefunction, |ψ(x)|^2, gives the probability density to find the particle at position x.
The "states" referred to in the Pauli Exclusion Principle are quantum states. These are complete descriptions of a system in quantum mechanics, typically represented as vectors in a complex vector space known as Hilbert space. A quantum state can be described by a set of quantum numbers, such as energy, angular momentum, etc.
Now, in a strong gravitational field like in a black hole, the curvature of spacetime becomes extremely large. In this regime, the concepts of "particle" and "state" become less clear. This is because the presence of a strong gravitational field can cause particle creation and annihilation processes, as described by quantum field theory in curved spacetime. In this case, what we normally think of as "particles" can be created and annihilated, and the notion of a fixed "state" becomes less well-defined.
As for equations, the description of particles in curved spacetime is typically done using quantum field theory in curved spacetime. This theory is described by equations like the Klein-Gordon equation or the Dirac equation in curved spacetime, which describe the behavior of quantum fields in the presence of gravity.
A very basic form of the Klein-Gordon equation in curved spacetime is:
□ψ + m^2ψ = 0,
where □ is the d'Alembertian operator in curved spacetime, m is the mass of the particle, and ψ is the quantum field.
However, these are highly complex theories that are still an area of ongoing research, and the details are far beyond a simple discussion or easy equations.
Furthermore, when it comes to black holes specifically, our current understanding is still incomplete, as it requires a theory of quantum gravity to correctly describe the physics, which we do not have yet. This is a major open problem in theoretical physics.
++++++
This is the end of the conversation, and I found it very satisfying. The reason that the Pauli Exclusion Principle is violated is not that the rule is “squishy” in any way…it is quite absolute. But rather, the intense distortion of spacetime fundamentally alters that force and energy and particles are. If you would like to know more about how intense gravity actually turns time momentum into spatial momentum, I would recommend that you also read my article Rivers of Spacetime.
I also found it fascinating that the Pauli Exclusion Principle doesn’t really take effect until the fermions are so crowded in energy-momentum space that all allowed energy states and momentum vectors are filled up or closed off. Basically, the potential energy of the fermions is battling the paradox that if you put them right on top of each other, both would disappear, violating conservations of energy, charge, spin, etc.
I also therefore found it fascinating that the effective per particle force of Degeneracy Pressure caused by the Pauli Exclusion Principle is around 10⁻¹¹ Newtons (Joules/meter) is similar to the rest mass energy of neutrons, which is about 1.5 x 10^-10 Joules (sorry, Substack does superscript zeroes really funny).
All in all, my two literal decades of puzzling over this have been satisfied about 80-90%. I need to think more about the answers GPT-4 gave me, and of course I need to find a way to check them to ensure they are even correct. But it seems they are, and this does answer a large part of the paradox I have been chasing. That said, it definitely does leave the door open for the hypothesis that in our universe, information plays a deeper role than simple labelling.